The world’s first magnetic induction cycler is now a registered medical device with CE-IVDR and TGA approval. Mic IVD is manufactured under an ISO 13485:2016 Quality Management System.
| Specs |
| Name | SKU | Size | Availability | Vendor | Price | Order | |
| Mic IVD qPCR Cycler | MIC-IVD | Generally 1-2 weeks from receipt of order | Bio Molecular Systems | Log in for pricing |
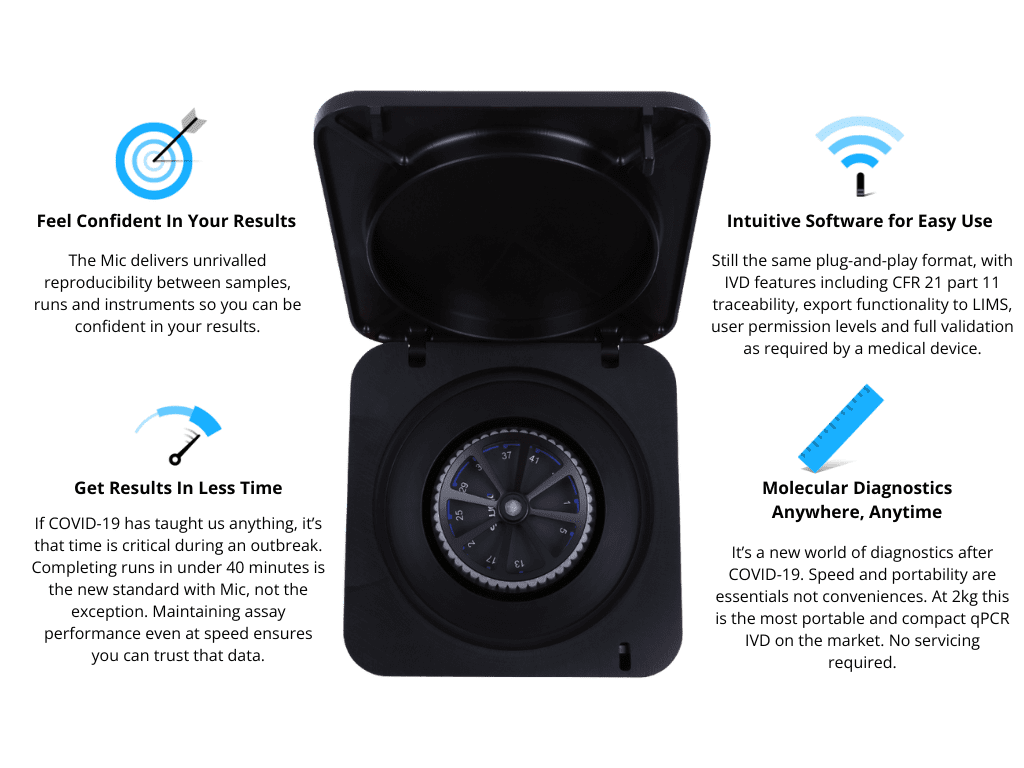
Get accurate results, quickly and easily, in the lab or on-the-go.

Never be frustrated with your results again. Engineered in our facility in Australia, the Mic IVD qPCR has served as an indispensable instrument for our partner facilities across the US, Europe, Africa, Latin America, and Asia. Integrating this instrument into your workflow will enable you to gather data and make a clinical diagnosis seamlessly.
TGA and CE-IVDR
| The Mic IVD instrument is intended to be used with CE-IVDR registered clinical diagnostic qPCR kits to provide detection of nucleic acid sequences in human-derived specimens. The Mic IVD instrument is intended for in-vitro diagnostic use by trained laboratory technicians and pathologists to interpret the results to make a clinical diagnosis. |  |
 |
Mic IVD | Specs
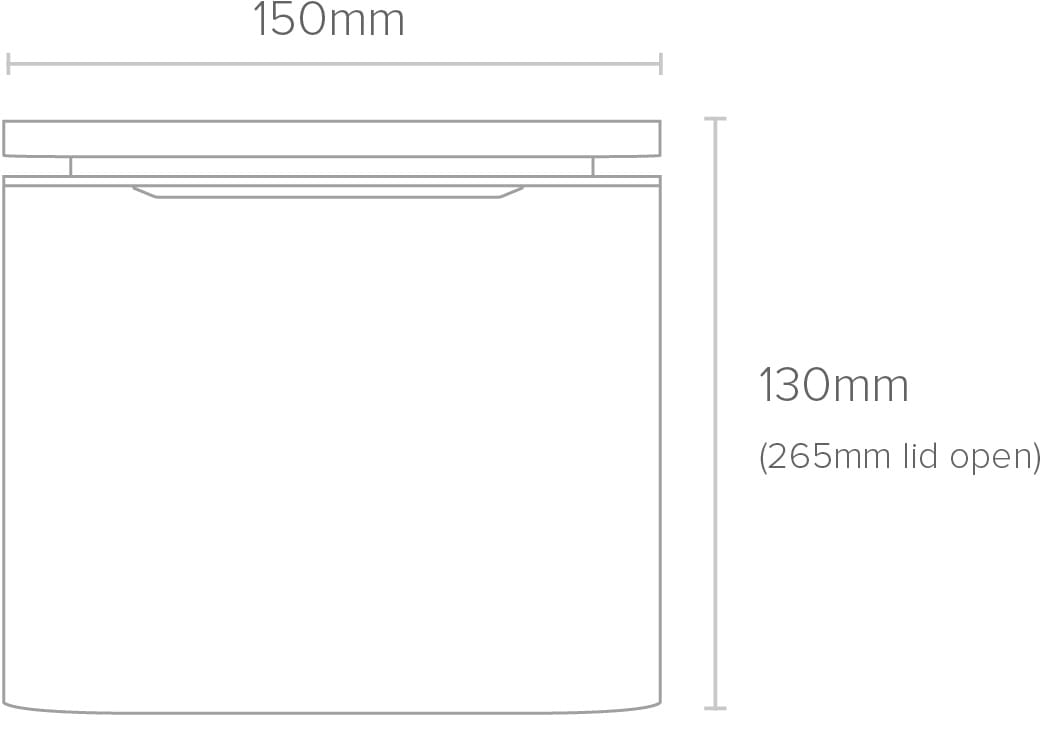
 Front Front |
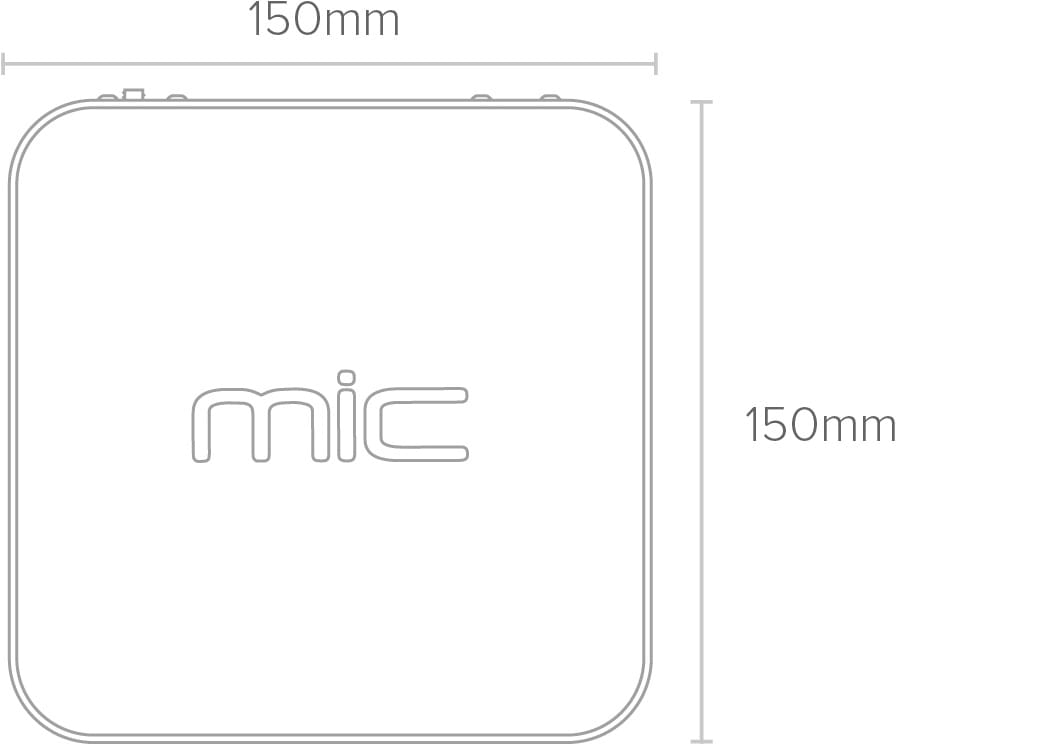
 Back Back |
| Physical |
|
|
| Thermal Performance |
|
|
| Optical |
|
|
| Reaction Tubes |
|
|
| Operating Environment |
|
|
| Transport and Storage Conditions |
|
|