Produce high yields of RNA transcript with this complete kit.
Key features
- Produce high yields of high quality RNA quickly and easily
- Synthesize long and short RNA with one kit
- Produce >20 times more RNA than conventional or “homemade” in vitro transcription kits
| Name | SKU | Size | Availability | Vendor | Price | Order | |
| AmpliScribe™ T7 High Yield Transcription Kit | AS3107 | 50 Rxns | Generally 1-2 weeks from receipt of order | LGC Biosearch Technologies - Lucigen (Epicentre) | Log in for pricing |
Product information
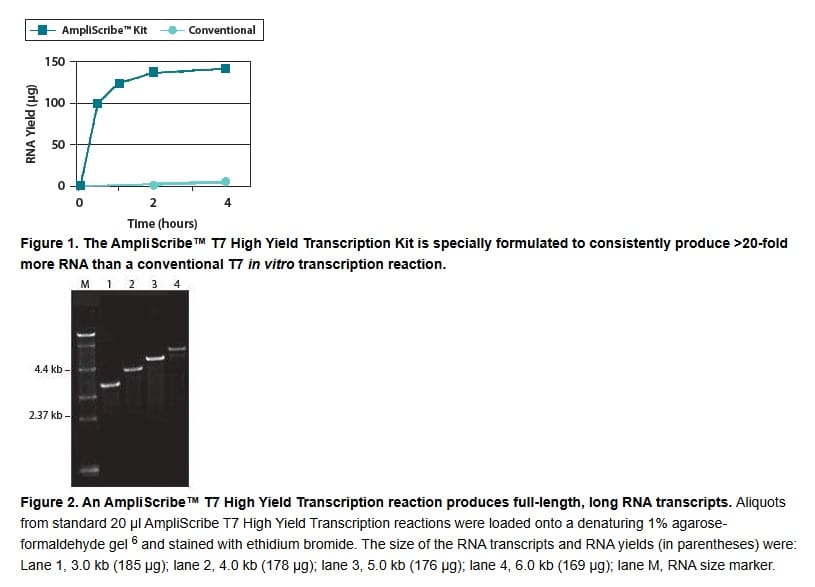
The AmpliScribe™ T7 High Yield Transcription Kit is specially formulated to use high concentrations of NTPs, that can be inhibitory to other in vitro transcription systems. AmpliScribe High Yield Transcription reactions can produce >20-fold more full-length RNA transcript than conventional in vitro transcription reactions. For example, the AmpliScribe T7 Kit yields approximately 5-7.5 mg/ml of 1.4-kb full-length control RNA in 30-120 minutes, respectively, using 1 µg of template DNA (Fig. 1).
Applications
- High-yield synthesis of RNA from DNA cloned downstream from a T7 RNA polymerase promoter, for microinjection, RNA interference, or antisense experiments.
- Structural and functional studies of splicing, processing, heterogeneous nuclear RNAs, tRNAs, viral RNAs, and ribozymes.1
- Production of fluorescent2-, biotinylated3-, or digoxigenin4– labeled RNA.
Features
- High yield. Produce microgram amounts of RNA from all templates—synthesize both long (Fig. 2) and short RNA using only one kit.5
- Sensitive. Produce microgram amounts of RNA from as little as 1 ng of template.
- Scaleable. Increase the reaction size to produce milligram amounts of RNA.
- Flexible. Substitute a labeled nucleotide for one of the supplied NTP solutions.
- Convenient. Rnase inhibitor included.
Note: Because of the very high concentration of NTPs used in AmpliScribe reactions, these kits are not recommended for preparing high specific-activity radiolabeled RNA probes.
References
- Johnson, M. (1995) Epicentre Forum 2(5), 4.
- DeLong, E.F. et al. (1999) App. And Environ. Microbiol. 65, 5554.
- Kaplan, E. et al. (1996) Epicentre Forum 3(2), 1.
- Hoff man, L.M. and Johnson, M.G. (1994) BioTechniques 17, 372.
- Schanke, J. (2000) Epicentre Forum 7(2), 6.
- Molecular Cloning – A Laboratory Manual, Third Edition, 2001. CSHL Press. pp 7.27 – 7.34. J. Sambrook and D. Russell.
Resources
Manuals and user guides
SDS