Aminooxy labelling can be used to conjugate glycoproteins after oxidation of the carbohydrates to generate aldhehyde groups.
Dyes functionalized with an aminooxy reactive group can be used to label molecules with a carbonyl group (aldehyde or ketone) to form a stable oxime bond. Aminooxy labeling can be used to conjugate glycoproteins after oxidation of the carbohydrates to generate aldhehyde groups. Aminooxy labelling of antibody glycosylation sites can be used as an alternative to succinimidyl ester labeling of amines, for antibodies where amine labelling affects the antibody binding affinity. Also see Biotium’s protocols for Succinimidyl Ester Labelling of Protein Amines and Maleimide Labelling of Protein Thiols.
The protocol below is a typical procedure for oxidation and aminooxy labelling of IgG antibodies. Protocols for labelling other carbonyl-containing molecules are similar, except that the oxidation step is not necessary for molecules with free aldehyde or ketone groups. In addition, the purification procedures may need to be modified for different labelled targets. The labelling reaction may be scaled up or down for any amount of protein as long as the ratios of the reagents are maintained.
| Materials required: | Workflow overview: |
|
|
Procedure:
1 – Prepare antibody solution for labelling
Dissolve the antibody in 1X PBS buffer, preferably at 20-100 uM (3-15 mg/mL for IgG). Low protein concentration may result in poor protein recovery yield or inefficient labelling.
2 – Oxidize antibody carbohydrate groups
2.1 – Prepare 100 mM sodium periodate (NaIO4) stock solution in dH2O.
2.2 – To the antibody solution from step 1, add 1/10 volume of 10X reaction buffer (1 M sodium acetate, 1.5 M NaCl, pH 5.5) and 1/10 volume of NaIO4 stock solution. For example, if you have 100 uL of protein solution, add 10 uL of 10X reaction buffer and 10 uL of NaIO4 stock solution.
2.3 – Incubate for 10 minutes at room temperature or 30 minutes on ice.
2.4 – Add ethylene glycol to the reaction to a final concentration of 100 mM to quench the periodate. The molarity of pure ethylene glycol is 14.5M, so add 0.69 uL of ethylene glycol for every 100 uL of reaction mixture. Incubate for 10 minutes at room temperature.
3 -Prepare the dye stock solution
Prepare a 5 mM stock solution of CF® Dye Aminooxy in water, DMSO or DMF. Aminooxy dyes are stable in solution and can be stored at -20°C, protected from light, for at least one year.
4- Carry out the labelling reaction
4.1 – Add 50 molar equivalents of CF aminoooxy reagent to the reaction from step 2. For example, to 100 uL of oxidized IgG at 50 uM add 50 uL of 5 mM dye stock. This corresponds to 5 nmol protein aldehyde and 250 nmol dye.
4.2 – Optional, but recommended: add 1/10 volume of Aniline, 10X in acetate buffer to catalyze the reaction. For example, if the mixture from step 4-1 is 150 uL in total, add 15 uL of 10X Aniline.
4.3 – Mix by vortexing the solution and incubate at room temperature with rocking or shaking for 2 hours, protected from light.
5 – Separate the labelled protein from the free dye
Prepare a Sephadex® column (10 mm x 300 mm) equilibrated in 1X PBS buffer. Load the reaction solution from step 3 onto the column and elute with PBS buffer. The first band excluded from the column corresponds to the antibody conjugate.
- For small scale labelling reactions, you may use an ultrafiltration vial to remove the free dye from the conjugate in order to avoid an overly dilute product. 10K MWCO can be used for IgG; proteins with different molecular weights may require different MWCO.
6 – Determination of degree of labelling (DOL)
Degree of labelling (DOL) is the average number of dye molecules conjugated to each antibody molecule. Measure the absorbance of the eluted antibody conjugate solution at 280 nm and at the absorption maximum of the dye used for labelling. The optimal range of DOL for each dye is listed in Table 1, although a DOL slightly above or below this range will also produce good results.
- The protein solution eluted from the column may be too concentrated for accurate absorbance measurement and thus must be diluted to approximately ~0.1 mg/mL. The fold of dilution (“dilution factor”) necessary can be estimated from the amount of starting antibody (i.e., 5 mg) and the total volume of the protein solution collected from the column.
6a. Determine the protein concentration
The concentration of the antibody conjugate can be calculated from the formula:
[conjugate] = {[A280 – (Amax x Cf)]/1.4} x dilution factor
where [conjugate] is the concentration in mg/mL of the antibody conjugate collected from the column; “dilution factor” is the fold of dilution used for spectral measurement; A280 and Amax are the absorbance readings of the conjugate at 280 nm and the absorption maximum respectively; Cf is the absorbance correction factor; and the value 1.4 is the extinction coefficient of IgG in mL/mg. See Table 1 for the Amax and correction factor for each CF® dye.
- If labelling a protein other than IgG, use the extinction coefficient for that specific protein.
- If using a dye other than a CF® dye, use the Amax and correction factor for that specific dye.
6b. Calculate the degree of labelling (DOL):
The DOL is calculated according to the formula:
DOL = (Amax x Mwt x dilution factor)/(ε x [conjugate])
The DOL is calculated according to the formula:
DOL = (Amax x Mwt x dilution factor)/(ε x [conjugate])
where Amax, “dilution factor” and [conjugate] are as defined in Step 6a, Mwt is the molecular weight of IgG (~150,000), and ε is the molar extinction coefficient of the dye (see Table 1).
- If labeling a protein other than IgG, use the molecular weight for that specific protein.
- If using a dye other than a CF® dye, use the extinction coefficient for that specific dye.
7 – Storage and handling of labelled antibody
For long-term storage, we recommend adding 5-10 mg/mL BSA and 0.01-0.03% sodium azide to the conjugate solution to prevent denaturation and microbial growth. The conjugate should be stored at 4°C, protected from light. If glycerol is added to a final concentration of 50%, the conjugate can be stored at -20°C. Under these conditions, antibody conjugates are stable for a year or longer.
Table 1: CF® Dye Technical Data
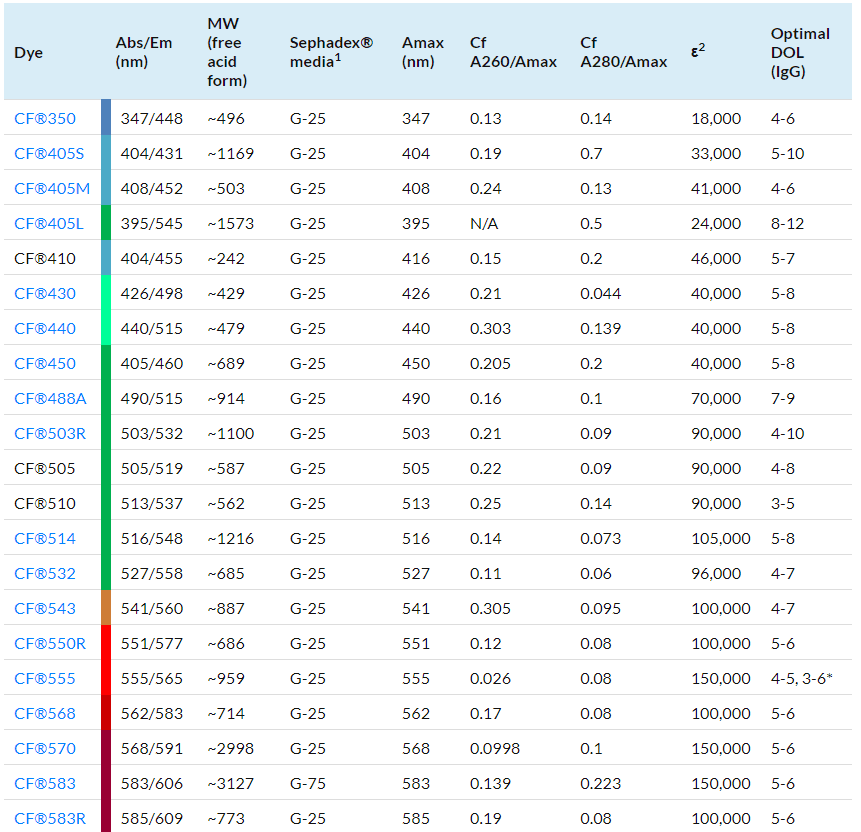
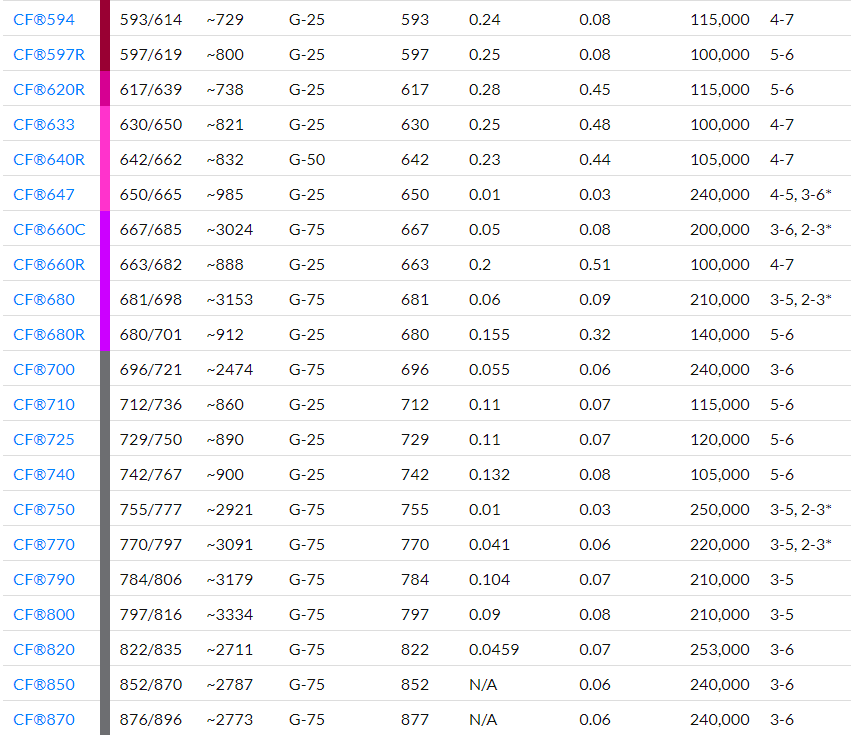
1. Sephadex® media recommendations are for antibody purification, not nucleic acid.
2. Extinction Coefficient (ε).
*Suitable, but suboptimal DOL.
Sephadex is a registered trademark of Cytiva.
For information on dye charge, download the CF® Dye Aminooxy Product Information Sheet.
