Membrane and cell surface stains are useful for visualizing cell borders and morphology in multicolor staining of live or fixed cells. Biotium offers several options for cell surface imaging, including the original CellBrite® Cytoplasmic Membrane Stains, which are specialized formulations of classic lipophilic carbocyanine dyes for membrane labeling. Since their release, Biotium scientists have designed a range of novel specialty membrane and cell surface stains for various applications:
|
 |
CellBrite® Fix Membranes Stains ORDER HERE |  |
MemBrite® Fix Cell Surface Staining Kits ORDER HERE |
 |
CellBrite® Cytoplasmic Membrane Dyes ORDER HERE |  |
CellBrite® Steady Membrane Staining Kits ORDER HERE |
 |
CytoLiner™ Fixed Cell Membrane Stains ORDER HERE |
To get the most out of our wide selection of membrane stains, check out these five tips for success:
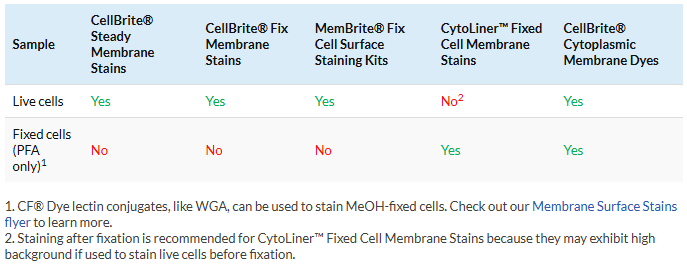
1. Choose the right stain for live or fixed cells
Original CellBrite® Cytoplasmic Membrane Stains can be used to stain live or formaldehyde (PFA)-fixed cells. CellBrite® Fix, MemBrite® Fix, and CellBrite® Steady should only be used on live cells. If they are used on cells after they are fixed, these dyes will mainly stain the cytoplasm or intracellular structures. CellBrite® Fix and MemBrite® Fix were created specifically to stain live cells immediately before fixation. CellBrite® Steady has been designed to image cell surface membranes of live cells for up to several days in culture. Cells stained with CellBrite® Steady retain both surface and intracellular staining over time. However, intracellular staining can be reduced or eliminated with the use of CellBrite® Steady Enhancer.
CytoLiner™ Fixed Cell Membrane Dyes were specifically developed for selective plasma membrane staining in PFA-fixed cells for microscopy. These dyes are engineered for robust and consistent staining of formaldehyde-fixed cells and are suitable for downstream immunofluorescence staining protocols.
For more help choosing a dye (plus information on using these dyes in bacteria or yeast), see our Tech Tip: Cell Surface Stains for Live & Fixed Cells, or download our Membrane & Surface Stains Selection Guide.
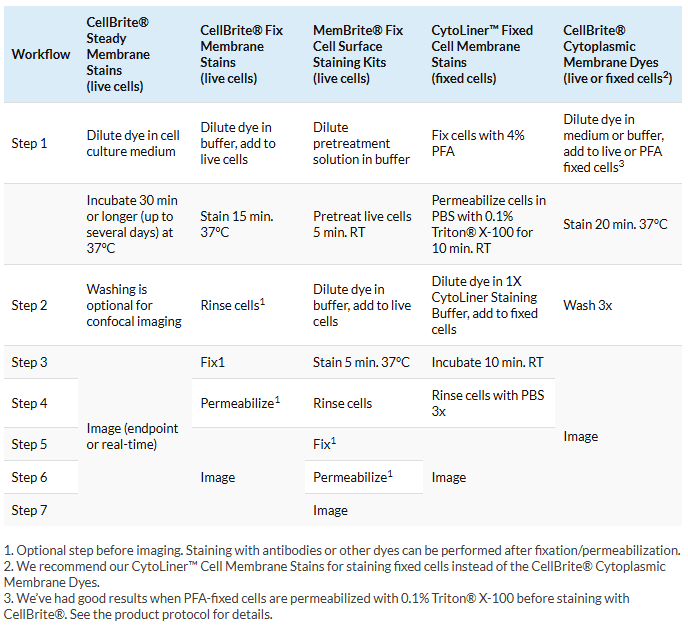
2. Understand the workflow before you begin
Each of our specialized membrane and cell surface staining products uses a different labeling protocol and has different requirements for downstream processing, such as fixation and permeabilization. Being familiar with the labeling protocol before you begin is a key step for getting good staining.
In general, we highly recommend making the dye solution immediately before staining for all membrane and cell surface staining protocols. Allowing the membrane dye solution to sit after diluting it may increase the occurrence of aggregates. Staining in complete medium may also decrease aggregation when using CellBrite® Cytoplasmic Membrane Dyes, regardless of whether you’re using fixed or live cells, as complete medium helps to solubilize the dye.
To get complete, step-by-step protocols, download the Product Information sheets:
- CellBrite® Green, Orange, & Red Protocol
- CellBrite® Blue Protocol
- CellBrite® NIR Protocol
- CellBrite® Fix Protocol
- CellBrite® Steady Protocol
- MemBrite® Fix Protocol
- CytoLiner™ Fixed Cell Membrane Stains
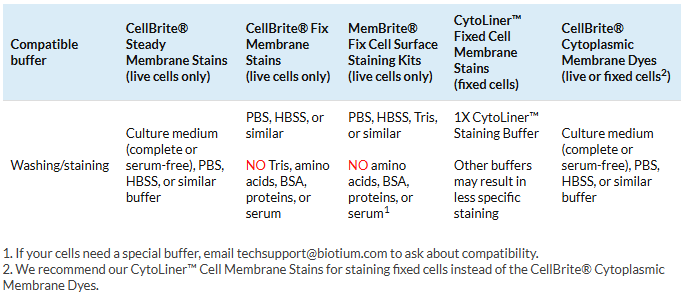
3. Use the right buffer for staining
Original CellBrite® Cytoplasmic Membrane Dyes and CellBrite® Steady Dyes can be added to cell culture medium with serum or buffers like PBS. However, CellBrite® Fix and MemBrite® Fix are chemically reactive compounds, so they have stricter buffer requirements. Make sure you’re using a compatible buffer to avoid interference with staining.
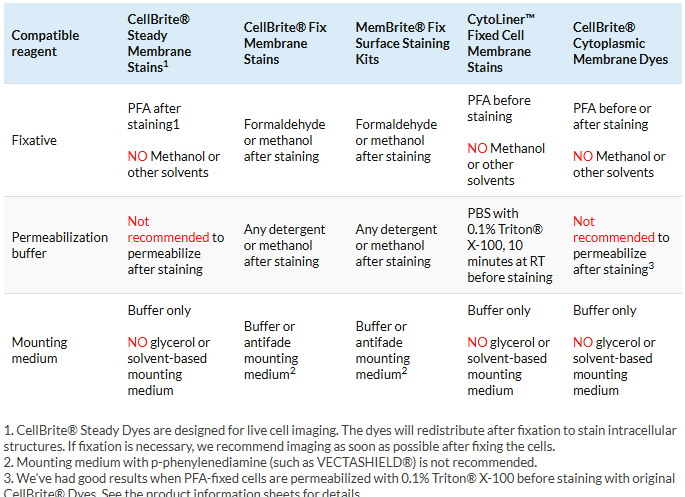
4. Use the right fixative and mounting medium
Original CellBrite®, CellBrite® Steady, and CytoLiner™ Dyes are compatible with formaldehyde (PFA) fixation. However, they can’t be used with solvents like methanol, detergents, or mounting medium with glycerol. In contrast CellBrite® Fix and MemBrite® Fix can be fixed, permeabilized, and mounted with any standard protocol. See below for guidelines on fixation, permeabilization, and mounting.
5. Know what to expect when imaging
Confocal vs. epifluorescence microscopy
If you have access to a confocal microscope, we recommend using it to image membrane staining for the best results. Confocal imaging screens out fluorescence from above and below the plane of focus, allowing very crisp imaging of cell boundaries. Compared to regular epifluorescence imaging, confocal is more sensitive and gives you more control over excitation power to limit photobleaching. Membrane dyes can be imaged with a regular epifluorescence microscope, but the images will be more diffuse because fluorescence from membranes above and below the cell borders will be captured.
Changes in dye localization over time in live cells
Original CellBrite® Cytoplasmic Membrane Dyes are very stable and have been reported to stain live cells for weeks in culture. However, dye localization in live cells changes over time. If live cells are cultured after staining, the labeled membrane will be internalized, so staining will gradually change from cell surface to intracellular vesicles, usually becoming mostly intracellular after about 24 hours.
CellBrite® Steady Dyes equilibrate between intracellular compartments and the plasma membrane. This allows the cells to retain surface staining as well as intracellular staining for several days. These kits also include CellBrite® Steady Enhancer as an optional reagent that can be used to mask intracellular fluorescence of CellBrite® Steady Dyes, providing more selective visualization of cell boundaries.
CellBrite® Fix and MemBrite® Fix localization in live cells will also change from cell surface to intracellular as membranes turn over, like original CellBrite®. Because they react with membrane proteins, staining with CellBrite® Fix and MemBrite® Fix is less stable in live cells than original CellBrite®. For this reason, we recommend fixing the cells immediately after staining with CellBrite® Fix or MemBrite® Fix stains.
Staining of dead cells
CellBrite® Fix, MemBrite® Fix, and CellBrite® Steady react irreversibly with cellular proteins. In live cells, this occurs on the cell surface because the dyes can’t penetrate the membrane. But they do get inside dead cells, where there are many more targets for reaction. As a consequence, the dyes stain dead cells much more brightly than live cells. For applications in which the cells will be stained after fixation, we strongly recommend using CytoLiner™ Fixed Cell Membrane Stains, which do not strongly stain dead cells. For protocols in which live cells will be stained and then fixed, our CellBrite® Cytoplasmic Membrane Dyes may be the best fit as they also do not brightly stain dead cells.
When imaging these stains, do not focus on very bright, rounded-up, or shrunken dead cells. Instead, imaging settings should be adjusted to detect cell membrane staining. The dead cell signal will likely be saturated under these settings. If the dead cell staining interferes with your imaging, try using high magnification and confocal imaging to exclude dead cells from the field of view. Or, try using one of our original CellBrite® Cytoplasmic Membrane Dyes, which do not show such dramatic differences in signal between live and dead cells.
Staining of fixed cells
CytoLiner™ Fixed Cell Membrane Dyes are optimized to stain plasma membranes in formaldehyde-fixed cells. The dyes will also stain tissue cryosections, but because plasma membranes are compromised by freezing and sectioning, the staining is not selective for the plasma membrane but will also stain intracellular structures. This stain cannot tolerate mounting medium or clearing agents, so cells should be imaged in PBS. CytoLiner™ signal is stable, so stained cells may be stored in PBS at 4°C, protected from light, for several days or longer prior to imaging. This stain is compatible with poly-L-lysine coated culture surfaces and Transwell® permeable supports. For best results, confocal microscopy is recommended for imaging fluorescent staining of Transwell® supports to avoid background from the filter material.
Original CellBrite® Cytoplasmic Membrane Dyes also may be used to stain formaldehyde-fixed cells. The staining can withstand permeabilization, but this can alter the dye localization and result in increased intracellular staining. Alternatively, we have seen good preservation of plasma membrane staining when cells are fixed with formaldehyde and then permeabilized before staining with the original CellBrite® Dyes. However, we strongly recommend using CytoLiner™ Fixed Cell Membrane Stains for staining formaldehyde-fixed cells because they offer more robust and consistent results over the CellBrite® Cytoplasmic Membrane Dyes.
Triton is a registered trademark of The Dow Chemical Company. VECTASHIELD is a registered trademark of Vector Laboratories.
